Heart disease is the leading cause of death for women. Women often experience heart disease differently than men. For example, men have more heart attacks than women, but women have a higher heart attack death rate. Women experience higher bleeding rates during percutaneous coronary interventions (PCI) performed through femoral arterial access. Women are also more susceptible to drug-induced cardiac arrhythmias.
FDA’s Office of Women’s Health (OWH) supports research to provide valuable insight into sex differences in the diagnosis and treatment of cardiovascular disease. OWH has worked across several FDA Centers to support studies on issues ranging from sex differences in cardiac interventions to the cardiotoxicity of breast cancer drugs. Since 1994, OWH has funded 69 studies (15 ongoing and 54 completed). The results of the completed studies have led to a better understanding of cardiovascular disease in women and contributed to the development of guidance documents for drug and device development for men and women.
· Current OWH-funded Research on Heart Disease
· Completed OWH-funded Research on Heart Disease
QT Prolongation: Research on the Effects of Drugs on Women’s Hearth Health
The FDA Office of Women’s Health has been a leader in supporting research to better understand and predict drug-induced heart arrhythmias in women.
Heart rate is controlled by electrical signals that pass through the heart each time it contracts and relaxes. These signals make up the heart’s electrical cycle – which is commonly measured by the waves on an electrocardiogram (ECG).
When the heart’s electrical cycle is abnormal, this can cause irregular heart rhythms (arrhythmias). A type of arrhythmia more common in women than in men is Torsade de Pointes (TdP). TdP events are rare but dangerous and can lead to sudden death. Several drugs have the potential to cause TdP and up to 70% of the drug-induced cases of TdP occur in women.
Almost all drugs that cause TdP prolong the QT-interval on the ECG (which corresponds to the heart’s relaxation phase). Since TdP events are rare, the drug-induced prolongation of the QT-interval on the ECG is used as an indicator for increased risk of TdP.
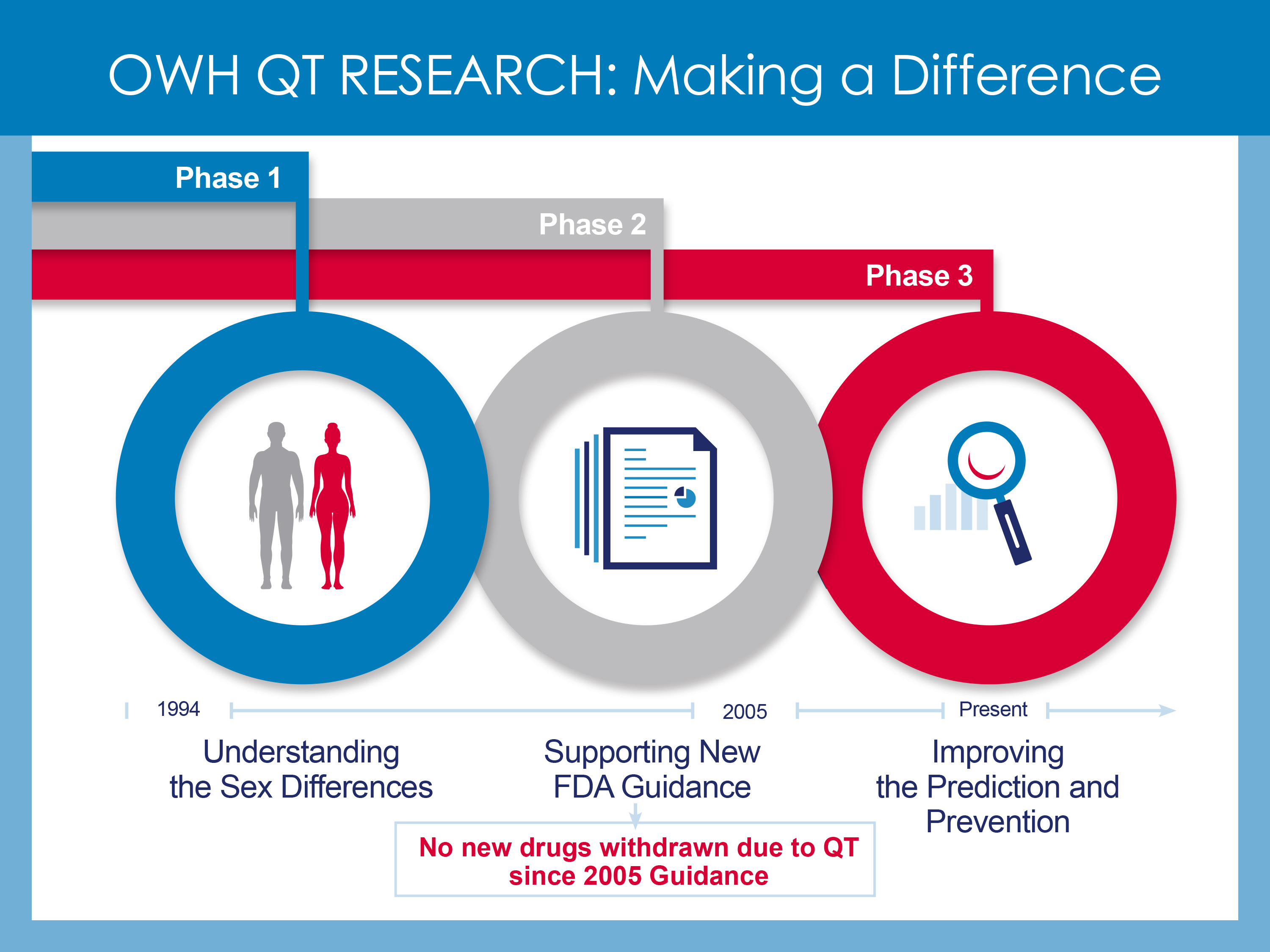
Since its inception in 1994, the Office of Women’s health has worked with FDA’s other research programs to support studies on drug-induced QT prolongation.

Phase 1: Understanding the Sex Differences
The exact reason for the higher rate of drug-induced TdP in women is unknown. However, a number of factors may play a role in this sex difference including higher drug levels in women due to smaller body size, influence of sex hormones, differences in how drugs are broken down and transported to the heart or greater sensitivity to drugs that cause abnormal heart rhythms. OWH funded studies to understand the mechanism of the sex differences in drug-induced QT prolongation. OWH also funded research within the Center for Drug Evaluation and Research (CDER) enabling post-market drug analysis to better recognize drug safety effects in women.
· Learn more about current OWH-funded research on QT and other CVD topics
· Learn about other FDA CVD research at the National Center for Toxicological Research
Phase 2: Supporting the Development of FDA Guidance
Building on the previous studies, OWH partially funded additional research on metabolic drug-drug interactions that contribute to QT prolongation. This research contributed to FDA guidance on the assessment of the QT prolongation potential of drugs for both men and women. As part of this guidance, FDA recommended that drug sponsors conduct a comprehensive study, called the Thorough-QT (TQT) study when seeking FDA approval of a new drug. The TQT study, implemented in 2005, has been an effective screening tool. Although certain commonly used drugs, such as antihistamines and antibiotics, had to be withdrawn from the US market because of drug-induced QT prolongation concerns, no such withdrawals have been necessary since 2005.
Phase 3: Improving Prediction and Prevention
Although the current FDA guidelines are very useful for identifying QT prolonging drugs, not all QT prolonging drugs cause TdP. OWH is currently partnering with CDER to sponsor studies to better screen for the subset of QT-prolonging drugs that have lower or no risks for TdP. This new research has the potential to enhance drug development and safety for both women and men by improving the accuracy of the evaluation of a drug’s potential to cause heart rhythm problems, and by providing this information earlier in drug development.
The information available on ewellnessmag.com, including text, graphics, and other materials are for informational purposes only. Reliance on any information in ewellnessmag.com is at the user’s own risk. The visitor of this web site acknowledges that the information available on or through the ewellnessmag.com is not and is not intended to be a substitute for professional medical advice.